research topics
Membrane fusion and the pre-hairpin intermediate
Despite over 3 decades of intense research, a prophylactic HIV/AIDS vaccine is not in sight. Most antibody-directed HIV-vaccine efforts target the native gp120/gp41 Env protein. In contrast, we are pursuing strategies to create novel HIV-1 vaccines that elicit antibodies against a therapeutically validated form of Env that is critical for HIV-1 infection – the pre-hairpin intermediate (PHI).
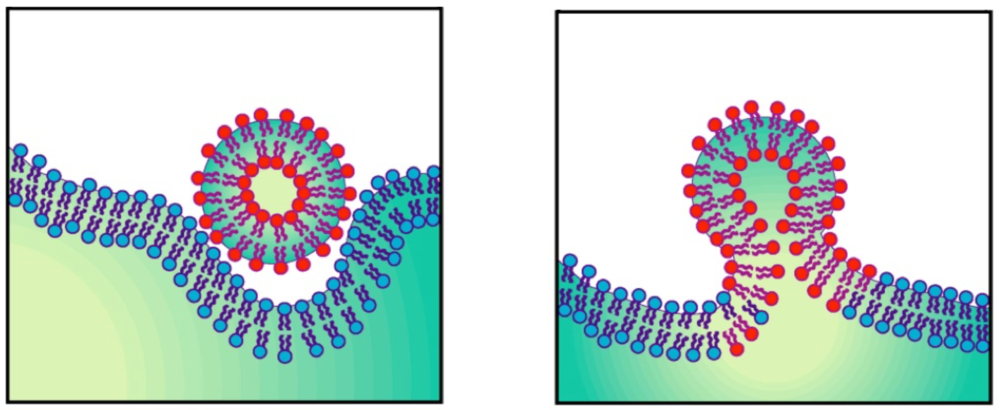
Enveloped viruses such as HIV-1 are characterized by a lipid bilayer that surrounds the nucleocapsid. To infect a cell, the membrane surrounding the virus must fuse with the membrane surrounding the host cell (Fig. 5). This membrane-fusion process is mediated by virally encoded, transmembrane-anchored glycoproteins. The mature HIV-1 envelope glycoprotein consists of two parts: a surface protein (SU) called gp120 and a transmembrane protein (TM) called gp41. On the virus surface, these glycoproteins exist as a trimer of gp120/gp41 heterodimers (Fig. 6).
Figure 5. Membrane fusion of an enveloped virus and its target cell.
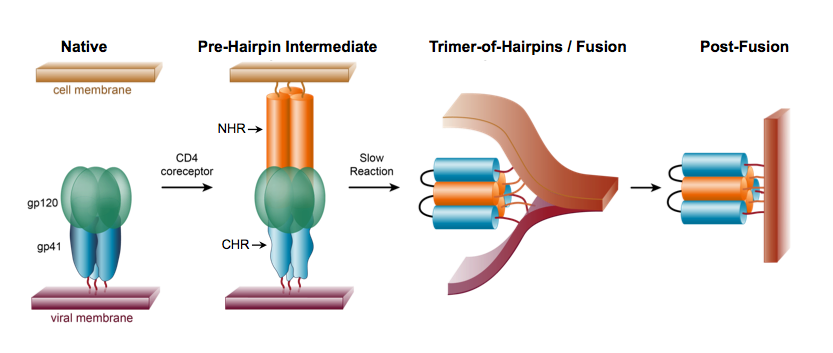
Figure 6. Schematic of membrane fusion mediated by the HIV-1 gp120/gp41 Envelope glycoprotein
Interactions between gp120 on the virus and CD4 receptors on target cells mediate attachment of HIV-1 to CD4+ T cells. This binding event induces a conformational change in gp120 that facilitates additional interactions between gp120 with co-receptors (CCR5 or CXCR4), leading to a dramatic “spring-loaded” conformational change in gp41 (Fig. 6). As a result, gp41 adopts a pre-hairpin intermediate (PHI) conformation, in which the protein is associated with two membranes simultaneously: the host cell membrane via the “fusion peptide”, and the viral membrane via the transmembrane domain (Fig. 6). Interactions between the N-heptad repeat (NHR) and C-heptad repeat (CHR) regions (orange and blue, respectively, in Fig. 6) lead to the formation of a “trimer-of hairpins,” or six-helix bundle, which brings the two membranes together.
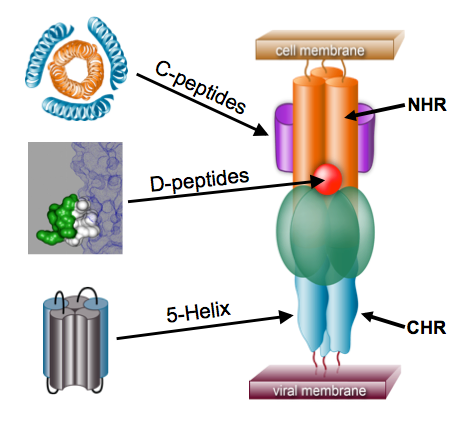
Figure 7. The pre-hairpin intermediate (PHI). The N-heptad repeat (NHR) and C-heptad repeat (CHR) regions are indicated. Different inhibitors work by binding to various regions of the PHI, thereby preventing formation of the trimer-of-hairpins. (review: Eckert & Kim [2001] Ann. Rev. Biochem.)
Peptides that bind to the NHR region of the PHI disrupt fusion by preventing formation of the trimer-of-hairpins. Such inhibitors include the C-peptides, derived from the CHR region (Fig. 7). Importantly, the FDA-approved HIV-1 drug enfuvirtide (Fuzeon™) is a C-peptide. Thus, the PHI is a validated drug target in humans.
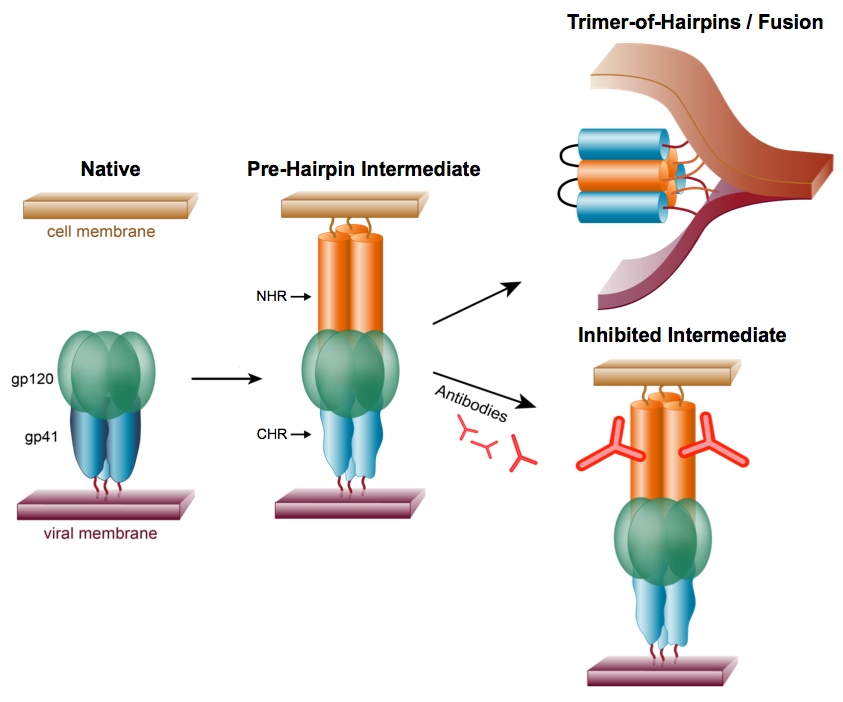
We aim to generate a vaccine that will elicit antibodies that, like enfuvirtide, will bind to the PHI and prevent HIV-1 infection by inhibiting formation of the trimer-of-hairpins (Fig. 8). An attractive feature of the NHR region of the PHI as a target for vaccine development is that it is very highly conserved among different HIV-1 strains, so antibody-escape mutants are predicted to be less frequent than for other regions of the gp120/gp41 envelope protein.
Figure 8. An HIV vaccine approach based on eliciting antibodies that bind to the pre-hairpin intermediate (Eckert et al. [1999] Cell).
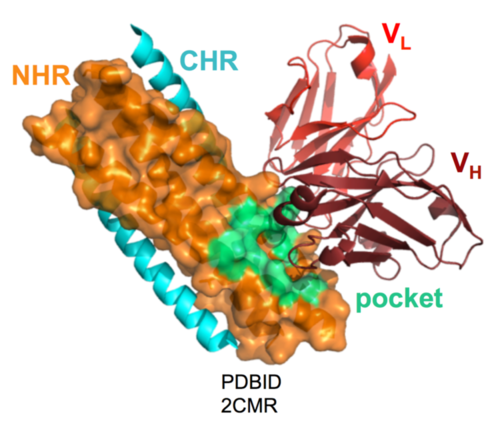
Because the PHI is transient, eliciting an immune response requires engineering of stable “mimetics” of the PHI to serve as immunogens. Using such PHI mimetics, we and others have elicited polyclonal antibody responses against the NHR region of the PHI. The resultant antisera inhibit HIV infection in cell culture, although the neutralization responses are weak. Monoclonal antibodies (mAbs) that bind to the gp41 NHR region and inhibit HIV-1 infection in cell culture have been isolated by us and others. Crystal structures for a few of these mAbs have been determined and, in each case, the mAb binds to a prominent pocket on gp41 (Fig. 9).
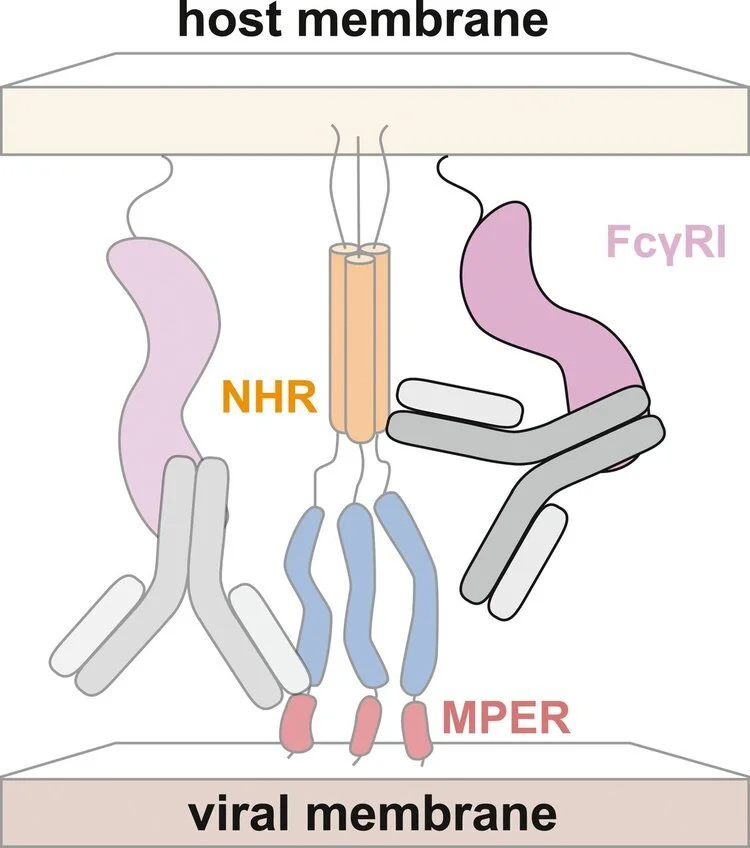
As with the polyclonal antibody responses, the neutralization potencies of these mAbs are generally weak. We showed that the neutralizing activity of one of these mAbs, D5, is potentiated >5000-fold in cells that express the immunoglobulin receptor FcγRI compared to those without (Fig. 10). Further, antisera from animals immunized with PHI mimetics neutralized HIV-1 effectively in an FcγRI-dependent manner. As FcγRI is expressed on macrophages and dendritic cells, which are present at mucosal surfaces and are implicated in the early establishment of HIV-1 infection following sexual transmission, these results may be important in the development of a prophylactic HIV-1 vaccine.
Figure 9. D5, an HIV-neutralizing antibody (Miller et al. [2005] PNAS), binds to the gp41 pocket (Luftig et al. [2006] Nature Struct. Mol. Biol.)
Figure 10. Hypothesized mechanism for FcγRI -mediated potentiation of antibodies targeting the NHR region of the PHI. The Fc domain of the antibody is bound by FcγRI , similar to the previously characterized mechanism of FcγRI -mediated potentiation of antibodies targeting the MPER.
publication highlights
Utilizing Machine Learning to Improve Neutralization Potency of an HIV1 Antibody Targeting the gp41 N-Heptad Repeat
(PDF) (ACS Chem. Biol.)
Maria V. Filsinger Interrante, Shaogeng Tang, Soohyun Kim, Varun R. Shanker, Brian L. Hie, Theodora U. J. Bruun, Wesley Wu, John E. Pak, Daniel Fernandez, & Peter S. Kim. ACS Chem. Biol. (2025). doi.org/10.1021/acschembio.5c00035.
A broad antibody with enhanced HIV-1 neutralization via bispecific antibody-mediated prepositioning.
(PDF) (Nat. Comm.)
Soohyun Kim, Caelan E. Radford, Duo Xu, Jianing Zhong, Jonathan Do, Dominic M. Pham, Katie A. Travisano, Maria V. Filsinger Interrante, Theodora U. J. Bruun, Valerie Rezek, Bailey Wilder, Martina Palomares, Michael S. Seaman, Scott G. Kitchen, Jesse D. Bloom & Peter S. Kim. Nature Communications. (2025). doi.org/10.1038/s41467-025-60035-6.
Structure-guided stabilization improves the ability of the HIV-1 gp41 hydrophobic pocket to elicit neutralizing antibodies. (PDF) (J. Bio. Chem.)
Theodora U. J. Bruun, Shaogeng Tang, Graham Erwin, Lindsay Deis, Daniel Fernandez, Peter S. Kim. Journal of Biological Chemistry (2023). doi: 10.1016/j.jbc.2023.103062.
Enhancing HIV-1 Neutralization by Increasing the Local Concentration of Membrane-Proximal External Region-Directed Broadly Neutralizing Antibodies. (PDF) (J Virol.)
Soohyun Kim, Maria V. Filsinger Interrante, Peter S. Kim. J Virol. (2022) 10.1128/jvi.01647-22.
HIV-1 prehairpin intermediate inhibitors show efficacy independent of neutralization tier. (PDF) (PNAS)
Benjamin N. Bell, Theodora U. J. Bruun, Natalia Friedland, Peter S. Kim. Proc. Natl. Acad. Sci. USA (2023). Feb 21;120(8) :e2215792120. doi: 10.1073/pnas.2215792120.
The high-affinity immunoglobulin receptor FcγRI potentiates HIV-1 neutralization via antibodies against the gp41 N-heptad repeat. (PDF) (PNAS)
David C. Montefiori, Maria V. Filsinger Interrante, Benjamin N. Bell, Adonis A. Rubio, Joseph G. Joyce, John W. Shiver, Celia C. LaBranche, and Peter S. Kim. Proc. Natl. Acad. Sci. USA (2021).
A derivative of the D5 monoclonal antibody that targets the gp41 N-heptad repeat of HIV-1 with broad tier-2 neutralizing activity. (PDF) (J Virol.)
Adonis A. Rubio, Maria V. Filsinger Interrante, Benjamin N. Bell, Clayton L. Brown, Celia C. LaBranche, David C. Montefiori, Peter S. Kim. J Virol. (2021) 95(15):e0235020.
Mechanisms of Viral Membrane Fusion and Its Inhibition. (PDF) (Annual Reviews)
Debra M. Eckert and Peter S. Kim. Annu. Rev. Biochem. (2001) 70: 777-810.